
FDA CDC News
FDA Outlines Steps to Strengthen Tobacco Program
Last Updated on February 24, 2023 by Daily News Staff
The U.S. Food and Drug Administration’s Center for Tobacco Products (CTP) outlined the steps it plans to take in response to an external evaluationExternal Link Disclaimer I commissioned last year from an independent panel of evaluators working through the Reagan-Udall Foundation. The evaluation was an important opportunity to take a critical look at the Tobacco Program’s regulatory processes and operations.
We have made tremendous progress preventing death and disability caused by tobacco use, but I am a strong believer that we can always benefit from examining how we can work most effectively and proactively to protect public health, support our staff, and be as responsive as possible to external stakeholders. When I started my career in intensive cardiac care, hospitals were full of relatively young people with sudden death, heart attacks, strokes and cancer attributable to tobacco use. The effectiveness of the public health and medical communities to reduce the toll of tobacco products was enhanced when the FDA was granted the authority to regulate tobacco.
As we enter this era of declining use of combustible tobacco and continued innovation in the e-cigarette industry, the societal concerns are not subtle. Our ability to keep pace with these changes will depend on immediate, short-term and long-term actions the center is taking that we believe will position the agency to more successfully implement our regulatory oversight of tobacco products.
CTP Director Brian King, Ph.D., M.P.H., has provided more detail about our approach to respond to the evaluation recommendations and our new plans, which will include the release of a 5-year strategic plan and comprehensive policy agenda by the end of the year. CTP has committed to providing regular updates on the progress of these activities, including some noted here.
Application Review
In the past several years in particular, CTP has made important progress in the review of applications for e-cigarette products – authorizing several tobacco-flavored e-cigarette products and devices and rejecting marketing applications for millions of products that did not meet the requirements in the law.
Given the ever-evolving tobacco marketplace, it is imperative that we optimize the framework for application reviews to ensure any products marketed meet the law’s public health and regulatory standards. This work will include, among other things:
- further streamlining of reviews when appropriate;
- increasing the use of the Tobacco Products Scientific Advisory Committee to discuss broader scientific matters central to premarket evaluation and individual product applications;
- posting current and future scientific policy memos and reviewer guides when appropriate; and
- working internally and through engagement with external stakeholders to better communicate on scientific issues and practices to support efficiency, effectiveness, and transparency. These critical efforts will be bolstered by a new director in CTP’s Office of Science, who will begin in late March.
To achieve these goals, we need to have the appropriate resources to hire and retain staff with the skills needed to effectively meet our public health mandate around tobacco. Since the agency’s fiscal year 2020 budget request, the FDA has advocated for the authority to collect user fees from e-cigarette companies, which currently do not pay user fees despite the enormous workload to review and make decisions regarding these products. The FDA also continues to explore ways, including engaging with the U.S. Department of Health and Human Services and the Office of Personnel Management, to identify solutions to facilitate more timely and efficient hiring of qualified and diverse professionals that match CTP’s needs.
Compliance and Enforcement
As the agency continues to make progress on its review of new and pending applications for novel products like e-cigarettes, the FDA will take additional action to remove illegal products from the market—particularly ones that have led to e-cigarettes being the most commonly used tobacco product among youth.
Between January 2021 and February 17, 2023, the FDA has issued more than 550 warning letters to companies for continuing to sell e-cigarette products that lacked the required FDA marketing authorization. These companies had millions of products listed with the FDA. After receiving warning letters, a majority of these companies have complied and removed their products from the market. However, in cases where companies did not do so, the FDA can pursue further enforcement action. For example, the FDA recently worked with the Department of Justice (DOJ) to file the first complaints for permanent injunctions against six e-cigarette manufacturers. Additionally, for the first time, the FDA this week filed civil money penalty complaints against four tobacco product manufacturers for manufacturing and selling new e-liquids without marketing authorization. The agency will continue to work as expeditiously as possible to remove illegal products from the market while identifying new ways to strengthen compliance and enforcement.
As recommended by the external evaluation, effective immediately, the FDA will begin planning to convene a summit with senior officials from the HHS and DOJ related to enforcement. The agency will also continue to work with other government agencies, such as U.S. Customs and Border Protection, the U.S. Postal Service, the Federal Trade Commission, Bureau of Alcohol, Tobacco, Firearms and Explosives, as well as with our compliance and enforcement partners at the state/local/territorial/tribal levels. This work will maximize compliance and enforcement activities where there are shared interests. Additionally, the FDA will explore alternative approaches to achieve compliance outside of judicial enforcement actions.
The agency acknowledges that some unlawful e-cigarette products continue to be sold in the U.S. Many companies have challenged the agency’s marketing denial orders in courts around the country. In some cases, this has constrained our ability to remove these products from the market while the legal cases are pending. It is also important for the public to understand that the agency does not have the authority to independently bring legal cases seeking injunction or seizure against those who violate the law. Instead, the agency must rely on DOJ, who must evaluate the legal risks of pursuing particular enforcement actions and decide whether to dedicate its finite resources to litigate cases on our behalf. The FDA will continue to use all the tools at our disposal to hold companies accountable; however, the agency cannot be everywhere at all times. The agency needs additional resources to ensure that companies comply and that we have the ability to take action against those who break the law and tie up the system in court proceedings.
Transparency and Communication
As part of the center’s work, the agency plans to enhance and increase its public communications to provide greater transparency about the agency’s approach to compliance and enforcement. By this spring, we plan to begin posting content to a new comprehensive webpage for all enforcement activities, which will include a searchable public database of all tobacco products that have an FDA marketing order to discourage the sale of illegal products. Additionally, the center plans to conduct additional public meetings and workshops, as well as provide more information regarding the application review process.
The center will also explore new ways for soliciting and considering public input on its public education campaigns, including input on formative research into messaging for adult smokers that nicotine – while highly addictive – is delivered through products that represent a continuum of risk.
The State of Tobacco Regulation in the U.S.
When Congress tasked the FDA with regulating tobacco products more than 13 years ago, the vision was to make tobacco-related disease and death part of America’s past, not America’s future, and, by doing so, ensure a healthier life for every family.
We’ve made notable progress, but important opportunities and challenges lie ahead as we seek to regulate an evolving marketplace. Current cigarette smoking among U.S. adults declined from 20.6% of the population in 2009 to 11.5% in 2021. Despite this progress, nearly 500,000 Americans still die every year from cigarette smoking and continued youth vaping is producing another generation plagued with addiction to products with unknown long-term health consequences.
We’ve announced plans to prevent initiation and help people quitExternal Link Disclaimer the deadliest form of tobacco use – combustible tobacco products. The agency is working to finalize product standards that would ban menthol in cigarettes and characterizing flavors (including menthol) in cigars. We’ve also announced plans for future potential regulatory actions, including developing a proposed product standard that would establish a maximum nicotine level to reduce the addictiveness of cigarettes and certain other combusted tobacco products. These actions are key to achieving the Cancer Moonshot goal of cutting the cancer death rate in half over the next 25 years, a key pillar of President Biden’s Unity Agenda.
Additionally, we continue to make significant strides in our science-based review of more novel tobacco products, such as e-cigarettes. The FDA has authorized 23 tobacco-flavored e-cigarette products and devices. The agency has also issued marketing denial orders for more than one million flavored e-cigarette products that lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health concerns posed by the well-documented, alarming levels of youth use of such products. While certain e-cigarettes may help adult smokers transition completely away from, or significantly reduce their use of more harmful combusted cigarettes, the law’s public health standard balances that potential with the known and substantial risk with regard to youth appeal, uptake, and use of these highly addictive products.
Unfortunately, the tobacco industry has fought the agency on many of the science-based actions we’ve taken – putting profits over public health. For example, despite being the only country with sweeping regulatory authority over the industry, unlike many other nations, legal challenges have twice prevented us thus far from implementing Congressionally mandated warnings on cigarette packs depicting the serious health risks of cigarette smoking. And, as noted earlier, many decisions about product applications have also been challenged in court, which has, among other things, required significant resources to defend.
The FDA will continue to undertake our critical work to improve public health. It’s imperative that we are able to meaningfully implement transformational regulations and make decisions based on the public health standard in the law, with the American public – not the interests of the tobacco industry – at the forefront. We’ve made progress, but there’s a lot more work to come.
Source: FDA
Discover more from Daily News
Subscribe to get the latest posts sent to your email.
Women's Health
Is Hormone Replacement Therapy Safe? What the FDA’s New Decision Means for Menopause Treatment
For more than 20 years, hormone replacement therapy for menopause has carried a warning label from the Food and Drug Administration describing the medication’s risk of serious harms – namely, cancer, cardiovascular disease and possibly dementia.
Last Updated on November 19, 2025 by Daily News Staff

Our Lifestyle section on STM Daily News is a hub of inspiration and practical information, offering a range of articles that touch on various aspects of daily life. From tips on family finances to guides for maintaining health and wellness, we strive to empower our readers with knowledge and resources to enhance their lifestyles. Whether you’re seeking outdoor activity ideas, fashion trends, or travel recommendations, our lifestyle section has got you covered. Visit us today at https://stmdailynews.com/category/lifestyle/ and embark on a journey of discovery and self-improvement.
Discover more from Daily News
Subscribe to get the latest posts sent to your email.
News
Nationwide Shrimp Recall Expands to Arizona: What You Need to Know
Nationwide Shrimp Recall: AquaStar has recalled Kroger, Kroger Mercado, and AquaStar frozen shrimp in Arizona and other states due to possible cesium-137 contamination. Check UPCs, lot codes, and best-by dates to see if your shrimp is affected.
Last Updated on September 26, 2025 by Daily News Staff
Steamed shrimp on plate
Nationwide Shrimp Recall Expands to Arizona: What You Need to Know
A major frozen shrimp recall is currently underway across the United States — and Arizona shoppers are directly affected. AquaStar (USA) Corp has announced a recall of multiple frozen shrimp products, both raw and cooked, due to potential contamination with cesium-137 (Cs-137), a radioactive substance.
Products Included in the Recall
The recall covers several popular brands and package types, including:
Kroger Raw Colossal EZ Peel Shrimp (2 https://stmdailynews.com/cash-trapping-how-to-protect-yourself-from-this-sneaky-atm-scam/ bag)
Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp (2 lb bag)
AquaStar Raw Peeled Tail-On Shrimp Skewers (1.25 lb bag)
AquaStar Cocktail Shrimp trays (sold at Walmart and other retailers)
In total, more than 85,000 packages of shrimp have been pulled from stores nationwide. These products were distributed to several states, including Arizona, between June and September 2025.
Why the Recall?
Routine testing detected the presence of cesium-137, a radioactive contaminant. While no illnesses or adverse reactions have been reported, long-term exposure to Cs-137 may increase the risk of certain cancers. Out of caution, the FDA and AquaStar urge consumers not to eat these shrimp.
🔍 How to Identify the Recalled Shrimp
Shoppers should look at UPC codes, lot codes, and best-by dates printed on the packaging. Here are the specific products under recall:
Product | UPC | Lot Code(s) | Best-By Date(s) |
|---|---|---|---|
Kroger Raw Colossal EZ Peel Shrimp (2 lb) | 20011110643906 | 10662 5085 10 · 10662 5097 11 · 10662 5106 11 · 10662 5107 10 · 10662 5111 11 · 10662 5112 10 · 10662 5113 10/11 · 10662 5114 10/11 | March 26 2027 · April 7 2027 · April 16–24 2027 |
Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp (2 lb) | 011110626196 | 10662 5112 11 · 10662 5113 10 | October 22–23 2027 |
AquaStar Raw Peeled Tail-On Shrimp Skewers (1.25 lb) | 731149390010 | 10662 5127 10 · 10662 5128 11 · 10662 5133 11 · 10662 5135 10 | November 7–15 2027 |
AquaStar Cocktail Shrimp Trays (Walmart) | 19434612191 | 10662 5106 · 10662 5107 · 10662 5124 · 10662 5125 | Dates vary by lot |
What Should Consumers Do?
Check your freezer for the affected shrimp products.
Do not eat them. If you have the recalled shrimp, throw it away or return it to the store where it was purchased.
Stay updated. The FDA continues to monitor the situation and will provide further updates as needed.
No Reported Illnesses So Far
Although the recall sounds alarming, health officials stress that no illnesses have been linked to these shrimp products at this time. The move is a precaution to protect consumers.
👉 Bottom line for Arizona shoppers: If you’ve bought frozen shrimp from Kroger, Kroger Mercado, or AquaStar between June and September 2025, check the packaging details immediately. When in doubt, don’t eat it.
🔗 Resources for More Information
FDA Recall Notice – AquaStar USA Corp Recalls Kroger & AquaStar Frozen Shrimp
FDA Advisory – Do Not Eat, Sell, or Serve Certain Imported Frozen Shrimp
STM Daily News
Cash Trapping: How to Protect Yourself from This Sneaky ATM ScamLink: https://stmdailynews.com/cash-trapping-how-to-protect-yourself-from-this-sneaky-atm-scam/
Discover more from Daily News
Subscribe to get the latest posts sent to your email.
Blog
Deadly ‘Kissing Bug’ Disease in Arizona: What You Need to Know
Learn about Chagas disease in Arizona, where kissing bugs are found, symptoms to watch for, treatment options, and prevention tips to protect your home and family.
Last Updated on September 8, 2025 by Daily News Staff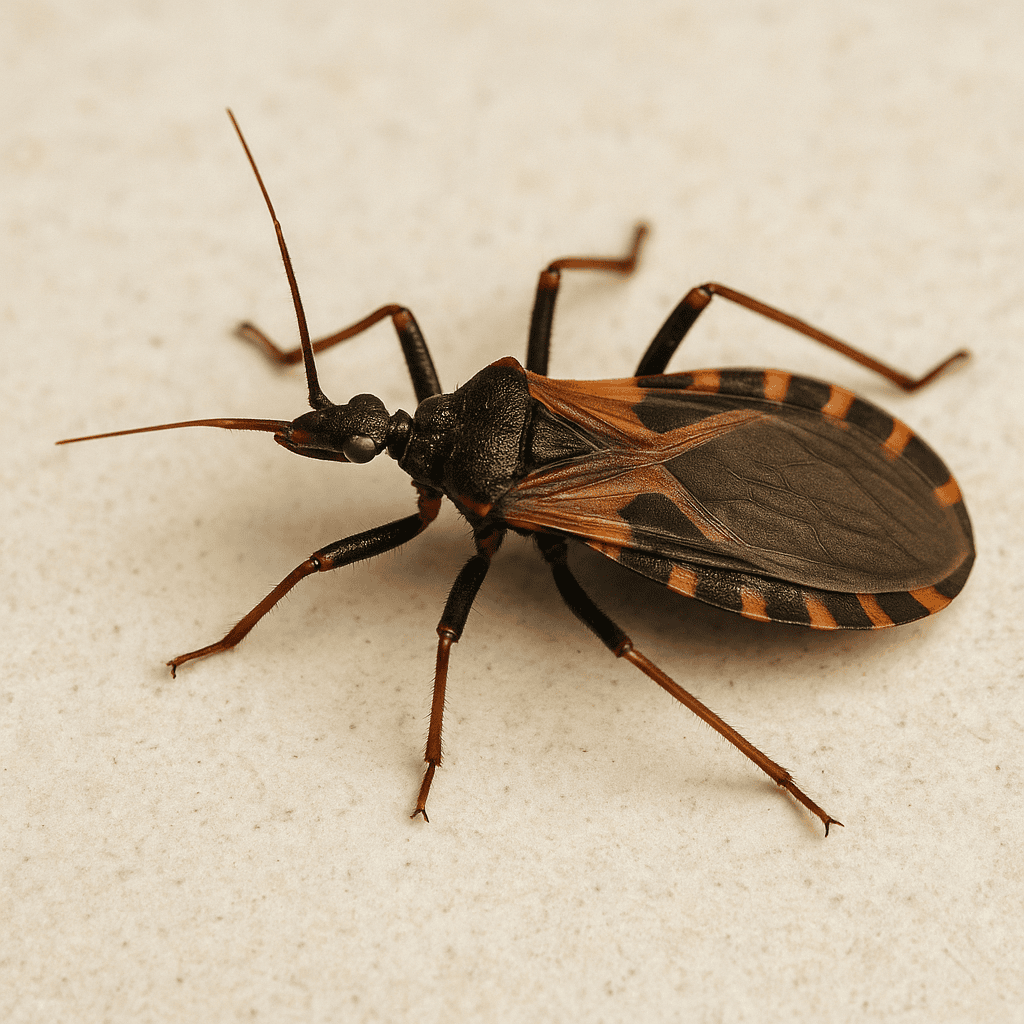
Chagas disease, often called the “kissing bug disease,” has been making headlines as it spreads in the United States. Arizona is one of the states where kissing bugs (Triatoma species) are common, particularly in the southern region. While confirmed human infections in Arizona have not been directly traced to bug bites, the insects are present, and many carry the parasite responsible for Chagas disease—making awareness and prevention critical.
Where Kissing Bugs Are Found in Arizona
Southern Arizona is a known hotspot for kissing bugs, with heavy activity in areas like:
Tucson and surrounding Pima County Cochise County Desert areas with packrat nests or outdoor animal enclosures
These insects are most active during late spring through early summer—from mid-May to mid-July—when they fly in search of food and shelter. Studies show that nearly half of the bugs collected in Arizona carry Trypanosoma cruzi, the parasite that causes Chagas disease.
How Kissing Bugs Spread Chagas Disease
Kissing bugs feed on the blood of humans and animals, often at night. Unlike mosquitoes, they don’t transmit the parasite through their bite itself. Instead, infection happens when:
The bug defecates near the bite wound and the parasite enters the skin through scratching. Contaminated bug droppings come into contact with the eyes, mouth, or open cuts.
Symptoms of Chagas Disease
Many people may not notice symptoms right away, but there are two phases of illness:
Acute Phase (weeks to months after infection)
Swelling or redness at the bite site Fever, fatigue, body aches Swollen eyelid (called Romana’s sign, a key indicator) Rash or loss of appetite
Chronic Phase (years later if untreated)
Heart problems (arrhythmias, enlarged heart, heart failure) Digestive issues (difficulty swallowing, severe constipation) Potentially life-threatening complications
If you suspect exposure, consult a doctor immediately. A blood test can confirm infection, and treatment is most effective when started early.
Treatment Options
Antiparasitic medications such as Benznidazole and Nifurtimox are available in the U.S. through the CDC. Treatment is most effective during the acute phase but may still help prevent complications in chronic cases. Doctors may also recommend heart or gastrointestinal monitoring for patients with chronic Chagas disease.
How to Prevent Kissing Bug Infestations
While human transmission in Arizona is rare, preventing bug exposure is the best protection.
Around Your Home
Seal cracks and gaps around doors, windows, roofs, and walls. Install and maintain window and door screens. Reduce outdoor lighting at night—bugs are drawn to light. Remove packrat nests, woodpiles, and debris near the home that can harbor kissing bugs. Keep pet sleeping areas clean and ideally indoors.
If You Find a Bug Indoors
Do not squash it with bare hands. Use a jar, plastic bag, or tissue to capture it safely. Freeze the bug or place it in rubbing alcohol for identification. Report findings to local health authorities or university research programs.
Key Takeaway
Southern Arizona—especially Tucson and surrounding counties—has a well-documented population of kissing bugs, many carrying the parasite that causes Chagas disease. While locally acquired human infections are rare, awareness and prevention are essential.
By sealing up your home, reducing nighttime exposure, and learning the signs of Chagas disease, you can greatly reduce your risk. If you notice unusual symptoms after possible exposure, don’t wait—get tested and treated early.
Related Links
CDC: Chagas Disease Information
University of Arizona Health Sciences:
Summertime Kissing Bug Season in Arizona
Texas A&M University: Kissing Bug Resource
National Library of Medicine: Chagas Disease in the United States
World Health Organization: Chagas Disease (American trypanosomiasis)
STM Daily News is a vibrant news blog dedicated to sharing the brighter side of human experiences. Emphasizing positive, uplifting stories, the site focuses on delivering inspiring, informative, and well-researched content. With a commitment to accurate, fair, and responsible journalism, STM Daily News aims to foster a community of readers passionate about positive change and engaged in meaningful conversations. Join the movement and explore stories that celebrate the positive impacts shaping our world.
Discover more from Daily News
Subscribe to get the latest posts sent to your email.
